By
Gabi Giacomin, Dr. of Naturopathic Medicine, Member Trisomy21 Research Foundation, SAC
What is 7,8 dihydroxyflavone?
7,8 DHF is a plant derived flavonoid found in a wide variety of fruits and vegetables. It is well established that flavonoids exert health benefits due to their antioxidant capacity. Recent evidence suggests they have the potential to improve memory by protecting brain cells (neurons), enhancing their function and stimulating brain cell regeneration. They also have a positive influence on LTP (long term potentiation) – one of the major pathways associated with learning, memory and cognitive performance.1
Using cell-based screening (looking at how cells respond to various substances), 7,8 DHF specifically bound to the TrkB receptor acting as an agonist, activating BDNF to enhance learning and memory and reduce stress and depression. 5
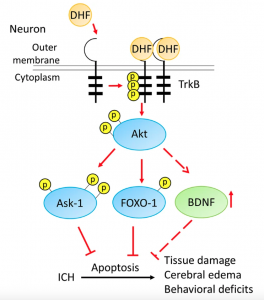
Diagram 1: 7,8 DHF attaches to the Trkb receptor activating BDNF
What is BDNF?
Proper brain development is dependant on the protein BDNF (brain derived neurotropic factor), whose primary role is to regulate the development and formation of brain cells. 7 BDNF also protects brain cells from glutamate toxicity (autism), reduces brain cell death (Alzheimer’s), injury from reduced blood flow, and improves the recovery of brain cells following traumatic brain injury.1
BDNF activates a number of neuronal populations including sensory neurons (associated with development of our five senses), motor neurons (required for movement), dopaminergic neurons (attention and learning) and cholinergic neurons (learning and memory, via acetylcholine). 1
Due to its brain enhancing effects, BDNF is considered a beneficial treatment for many aspects of the health and development of people with Down Syndrome (DS). 1 Trials on genetically manufactured BDNF have been disappointing due short term bioavailability and poor delivery, and as a result natural treatments are being examined 1,5

Diagram 2: Neuroplasticity
Prenatal Studies
Low amounts of BDNF have been detected in foetuses and mice with DS, indicating chronically low levels prenatally and throughout life. 7 Low BDNF levels contribute to poor development of sensory, motor, dopaminergic and cholinergic pathways making it a key driver of intellectual impairment in DS. 6
The period of gestation from week 7-20 is when the generation of new brain cells is prolific. This ceases when a child is born, except in the hippocampus and olfactory brain regions it continues throughout life. 6 As a result, scientists are targeting the regeneration of brain cells during the neonatal period. 6 Prenatal treatment with 7,8 DHF restored brain cells in the hippocampus (emotional memory recall and regulation) in DS mouse models with mild long-term effects. 6 In addition it increased the total number of excitatory neurons (granules) and sites of contact between brain cells (dendritic spine density). 7 However, if treatment during the neonatal period is discontinued, the benefits are lost. 6 In earlier studies, early postnatal treatment had the same effect on mice. 6
Trials of Fluoxetine (an antidepressant which enhances neurogenesis) given to DS mice prenatally, regenerated brain cells in a number of brain regions 6. However, the use of Fluoxetine during pregnancy has been linked to alterations in heart development, making its safety profile during the delicate fetal period tenuous. 6
More studies are needed to establish whether treatment with 7,8 DHF started in the neonatal period and continued in the early postnatal period are sustainable throughout life. 6
Mitochondria Studies
The generation of new brain cells depends on active cellular energy production. 7 Low energy levels reduce brain cell survival and activity and are associated with several diseases linked to intellectual disability including DS. 7
When scientists studied cells from a region of the DS brain associated with learning and memory (hippocampus) a link between poor energy and poor brain cell formation was detected. 7 This was reversed in previous studies using Resveratrol and EGCg.7 Resveratrol and EgCg activate cellular energy production via regulation of pathways linked to their function. 7 (article on Mitochondria + DS here)
Recent studies demonstrate that 7,8 DHF improves cellular energy levels in the brains of newborns with DS. 7 Between birth and puberty the maturing brain requires healthy energy levels to ensure a fast metabolic rate to satisfy brain development demands during this period. 7 The use of 7,8 DHF during foetal life and in newborns is not associated with adverse effects, and can be used safely in early life. 7
Enhancing energy levels in the brain has many benefits for people with DS including 1) a reduction in body weight through enhancing ATP production in muscle cells, 2) protecting the heart from toxicity 3) protecting the heart from damage caused by low energy 4) protecting the brain from injury 5) neurogenesis. 7
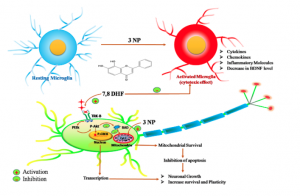
Diagram 3: 7,8 DHF inhibits inflammation from activated microglia (immune cells in the Central Nervous System – Brain) and stimulates BDNF via Trkb activating mitochondria, inhibiting cell death in the CNS (linked to AD) and increasing cell growth (neurogenesis).
Treatment with 7,8 DHF increased levels of PGC1a which not only increases cellular energy levels and respiration in skeletal muscle, but activates mitochondrial cleaning (mitophagy), transformation (remodelling) and restoration. 7
7,8 DHF activates the Nrf2 antioxidant pathway in cells increasing catalase and heme-oxygenase-1 (necessary for iron recycling) and reducing chronic oxidative stress linked to early ageing in people with DS. 14 ,15,16 Recent studies report that stabilisation of the Nrf2 pathway in people with DS is critical for maintaining cellular health, and therapies such as 7,8 DHF which target the mitochondria are key to preventing senescence (ageing). 14,15,16
Any therapy that improves energy levels in people with DS should be encouraged and started early in life to enhance brain development. Long term treatment with polyphenols (flavonoids) is recommended considering the benefits in newborns are short lived. 7
Alzheimer’s Disease
Reduced levels of BDNF occur in people with Alzheimer’s disease and are thought to play a role in its development. 1, 12 Implementation of BDNF in AD trials shows a protective role against AD development by increasing learning and memory in animals with dementia. 1, 12
Reduced levels of acetylcholine and choline are observed in patients with AD. 1 Currently medication to treat AD (cholinesterase inhibitors and NMDA antagonists) is designed to increase acetylcholine and inhibit glutamate overactivity, but only delay the progression of disease without addressing the underlying causes including plaque formation, neurofibrilary tangles and loss of neurons. 1
7,8 DHF blocks BACE1 activity (increased in DS brains) lowering the level of AB plaque formation in the brain of mice with AD gene mutations leading to improved memory. 1, 5, 7,8 7,8 DHF protects brain cells from AB plaque induced cell death and promotes the formation of brain cell dendrintes and synapses. 12
One of the mechanisms through which AB plaque causes dementia is through unregulated glutamate production in the brain. 13 Activation of BDNF protects the brain from glutamate toxicity. 2
Obesity
In addition to regenerating brain cells, BDNF stimulation regulates food intake and weight control. 4 Mice bred with low BDNF levels displayed overeating and obesity, while those given BDNF regulators had a reduction in food intake and weight gain.1, 4
BDNF acts as an energy metabolism regulator, acting on the brain and skeletal muscle, and is stimulated by exercise.1, 4
7,8 DHF administration didn’t reduce food intake, as BDNF does, but did significantly decrease body weight gain in animals fed a healthy diet or high fat diet (HFD), with a more striking effect when consumed in females on a HFD than malesl. 1, 4 7
Mice treated with 7,8 DHF also had improved insulin levels, lower blood glucose and improved insulin sensitivity in liver, fat and muscle tissue. It seems its effectiveness in treating diabetes is associated with reductions in obesity. 1
The ability of 7,8 DHF to regulate glucose levels reduces oxidative stress; this prevents damage to the nervous system in diabetics restoring associated neuropathies involving nerve conduction (sciatic and saphenous). 11
One important consideration is that the dose of 7,8 DHF be carefully determined, as too much can over excite brain cells leading to learning deficits from overstimulation 1
Traumatic Brain Injury
Traumatic brain injury (TBI) causes direct damage to brain tissue, as well as indirect damage. 8 Degeneration can occur in brain cells after injury, including sustained invisible damage even if they survive direct injury. 8
BDNF activation is associated with neuron survival, reproduction and plasticity following TBI, preventing brain cell degeneration. 8 Laboratory results reveal that pretreatment with 7,8 DHF prevents neuron death in the brain (cortex and hippocampus) caused by moderate TBI. 8 Post treatment with 7,8 DHF dramatically reduced damage to brain cell arms (dendrites), preventing degeneration and death, resulting in better motor function following TBI, but no significant improvement in learning and memory. 8 Future trials will continue to test duration, dose and combination of 7,8 DHF with other therapies. 8
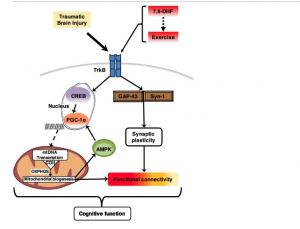
Diagram 4: 7,8 DHF enhances exercise post Tramatic Brain Injury
Eye Disorders
The use of 7,8 DHF has been studied in relation to eye disorders common to adults with DS, including diabetic retinopathy, macular degeneration, glaucoma, complications of glaucoma surgery etc. 9, 10 Its ability to inhibit VEGFR2 gene activity improves vision by decreasing inflammation in the eye 9, 10
Summary
The specific structure of 7,8 DHF has made it an ideal treatment for BDNF enhancement. It regulates the action of BDNF in the brain, enhancing learning and memory with anti-stress and anti-depressant effects. 5 It improves memory in animal models of AD, by reducing BACE1 activity, resulting in reduced alpha beta plaque. 5 There is no toxicity associated with long term treatment 5. It crosses the BBB (blood brain barrier) and is orally bioactive. Studies indicate 7,8 DHF holds great therapeutic efficacy for people with DS. 5
DOSING FOR ADULTS WITH DOWN SYNDROME:
Recommended dose: Adults with T21 ages 15 and up: 25 mg 2 x a day to increase to 3 x a day.
Prenatal dose: 25mg 2 x daily (not to exceed 100mg daily) to birth and then every 3 hours if desired.
Not currently recommended for children under 15 years of age as clinical trials are still underway. Stay tuned for updates on the ‘Targeted Nutritional Intervention’ MeWe group and ‘The Conscious Pod’ Public Favebook Group.
Australian Supplier
US/ AU Amazon
References:
1. Liu C, Chan CB, Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl Neurodegener. 2016;5:2. Published 2016 Jan 6. doi:10.1186/s40035-015-0048-7
2. Jang SW, Liu X, Yepes M, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687-2692. doi:10.1073/pnas.0913572107
3. Liu X, Chan CB, Jang SW, et al. A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J Med Chem. 2010;53(23):8274-8286. doi:10.1021/jm101206p
4. Chan CB, Tse MC, Liu X, et al. Activation of muscular TrkB by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem Biol. 2015;22(3):355-368. doi:10.1016/j.chembiol.2015.02.003
5. Chen C, Wang Z, Zhang Z, et al. The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. Proc Natl Acad Sci U S A. 2018;115(3):578-583. doi:10.1073/pnas.1718683115
6. Stagni F, Uguagliati B, Emili M, Giacomini A, Bartesaghi R, Guidi S. The flavonoid 7,8-DHF fosters prenatal brain proliferation potency in a mouse model of Down syndrome. Sci Rep. 2021;11(1):6300. Published 2021 Mar 18. doi:10.1038/s41598-021-85284-5
Valenti D, Stagni F, Emili M, Guidi S, Bartesaghi R, Vacca RA. Impaired Brain Mitochondrial Bioenergetics in the Ts65Dn Mouse Model of Down Syndrome Is Restored by Neonatal Treatment with the Polyphenol 7,8-Dihydroxyflavone. Antioxidants (Basel). 2021;11(1):62. Published 2021 Dec 28. doi:10.3390/antiox11010062
7. Zhao S, Gao X, Dong W, Chen J. The Role of 7,8-Dihydroxyflavone in Preventing Dendrite Degeneration in Cortex After Moderate Traumatic Brain Injury. Mol Neurobiol. 2016;53(3):1884-1895. doi:10.1007/s12035-015-9128-z
8. Chitranshi N, Gupta V, Kumar S, Graham SL. Exploring the Molecular Interactions of 7,8-Dihydroxyflavone and Its Derivatives with TrkB and VEGFR2 Proteins. Int J Mol Sci. 2015;16(9):21087-21108. Published 2015 Sep 3. doi:10.3390/ijms160921087
9. Flavia Messina, Andrea Cerquone Perpetuini, Husvinee Sundaramurthi, Ailis Moran, Milton English, Matthew Brooks, Anand Swaroop, Madhuri Dandamudi, Alison L Reynolds, Niall O’Reilly, Breandan N Kennedy; Evaluation of the neuroprotective mechanisms of 7,8-Dihydroxyflavone and generation of corresponding sustained release nano/microparticles for improved ocular drug delivery. Invest. Ophthalmol. Vis. Sci. 2021;62(8):1202.
10.Cho SJ, Kang KA, Piao MJ, et al. 7,8-Dihydroxyflavone Protects High Glucose-Damaged Neuronal Cells against Oxidative Stress. Biomol Ther (Seoul). 2019;27(1):85-91. doi:10.4062/biomolther.2018.202
11. Aytan N, Choi JK, Carreras I, et al. Protective effects of 7,8-dihydroxyflavone on neuropathological and neurochemical changes in a mouse model of Alzheimer’s disease. Eur J Pharmacol. 2018;828:9-17. doi:10.1016/j.ejphar.2018.02.045
12. Findley CA, Bartke A, Hascup KN, Hascup ER. Amyloid Beta-Related Alterations to Glutamate Signaling Dynamics During Alzheimer’s Disease Progression. ASN Neuro. January 2019. doi:10.1177/1759091419855541
13. Zamponi E, Zamponi N, Coskun P, Quassollo G, Lorenzo A, Cannas SA, Pigino G, Chialvo DR, Gardiner K, Busciglio J, Helguera P. Nrf2 stabilization prevents critical oxidative damage in Down syndrome cells. Aging Cell. 2018 Oct;17(5):e12812. doi: 10.1111/acel.12812. Epub 2018 Jul 20. PMID: 30028071; PMCID: PMC6156351.
14. Cai D, Feng W, Liu J, et al. 7,8-Dihydroxyflavone activates Nrf2/HO-1 signaling pathways and protects against osteoarthritis. Exp Ther Med. 2019;18(3):1677-1684. doi:10.3892/etm.2019.7745
15. Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88(Pt B):179-188. doi:10.1016/j.freeradbiomed.2015.04.036
 English
English Español de México
Español de México Português
Português Français
Français
Read